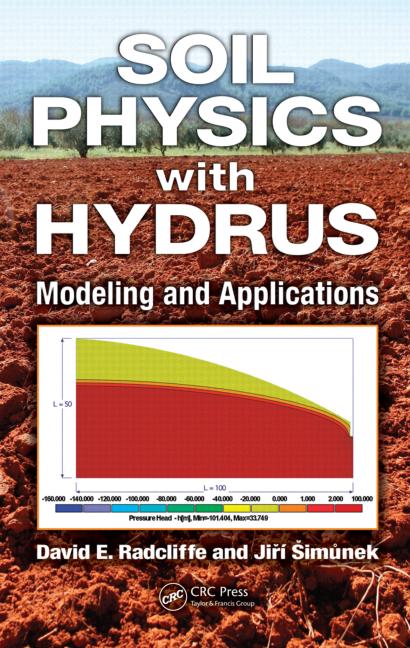
When do soil carbonates form? We need to be able to answer this question in order to use ancient soil carbonates for paleoclimate reconstructions.
It is commonly assumed that soil carbonates form in the warm season. This assumption is reasonable because the warm season is when plants are growing (and evapo-transpirating), the sun is shining, and the soil is drying out. All of those physical processes should promote supersaturation with respect to calcite.
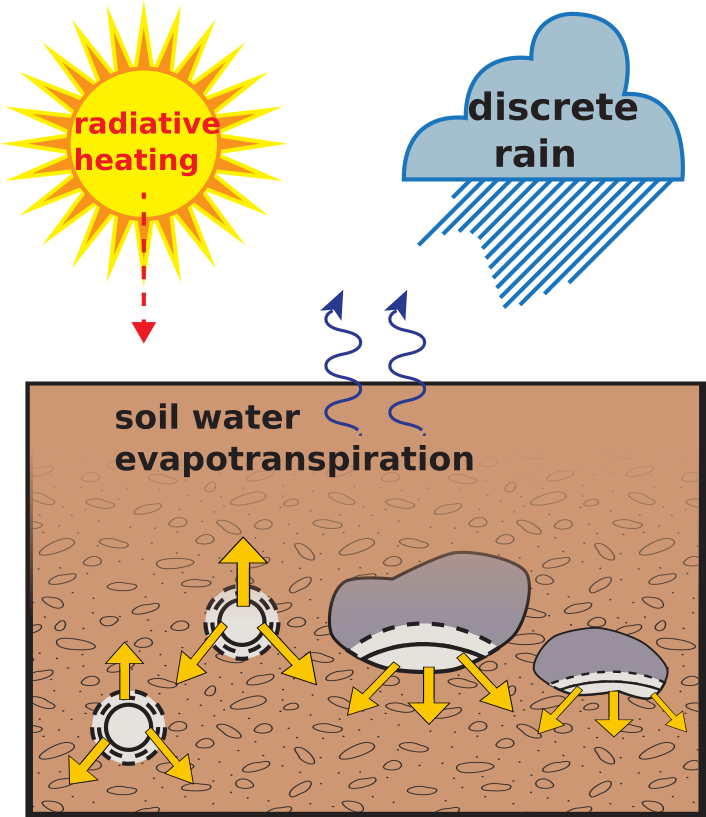
Yellow arrows indicate carbonate formation (nodules and pendants). Illustration help from Landon Burgener.
BUT soil carbonates can form in the winter, spring, summer, or fall. Environmental factors that control this timing include plant activity, persistent snowpack, soil texture, heavy summer rain, etc.
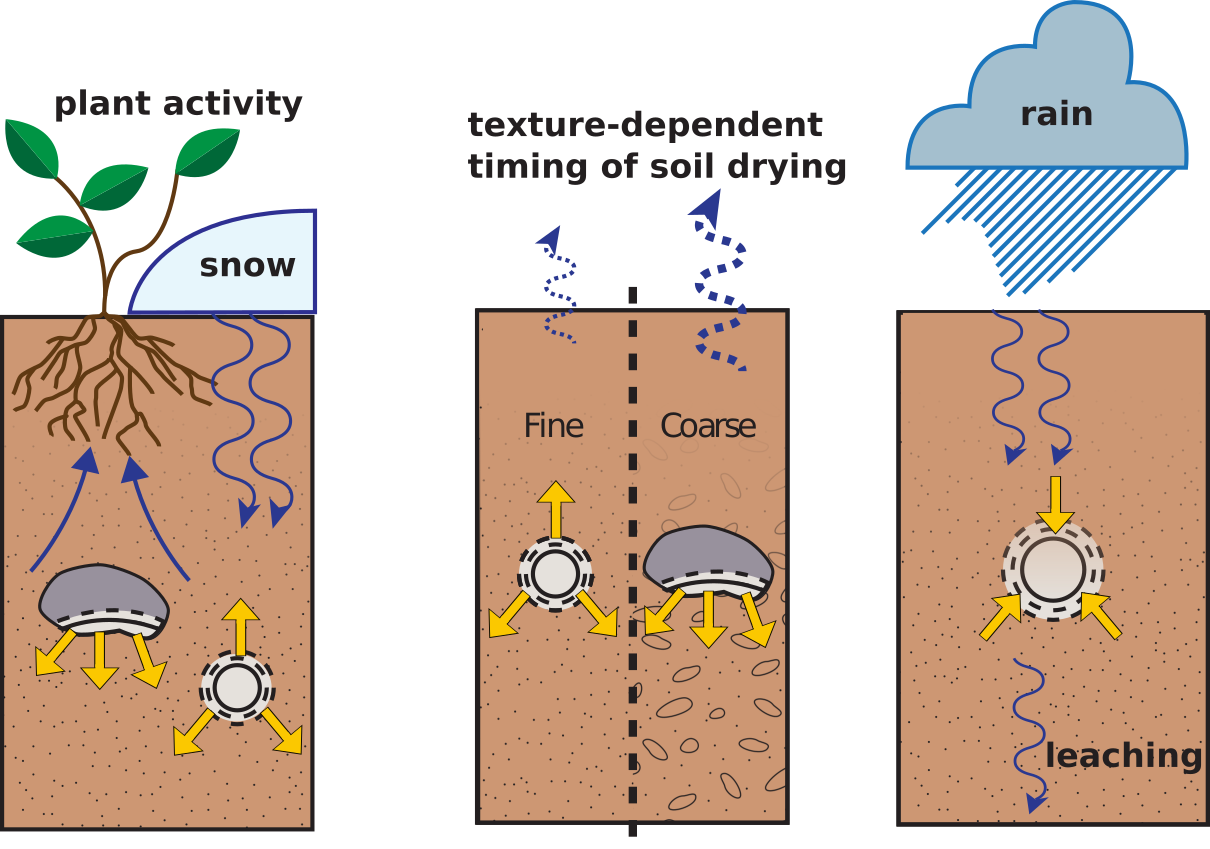
Environmental factors that can change the timing of carbonate formation.
I am using multiple approaches to determine when soil carbonates form and how to interpret their isotopic composition.
First, this paper reviews clumped isotope data on soil carbonates. Clumped isotope-based temperatures can reveal apparent seasonal biases in modern soil carbonates. The take home: soil carbonate formation temperatures are all over the place! A warm-season bias is the most common, but formation temperatures deviate widely from mean annual air temperatures. We aren’t able to explain this variation in formation temperatures easily with environmental data and existing hypotheses.
Next, I am using a numerical model called HYDRUS-1D to predict the timing of carbonate formation. This work will allow us to delve into individual processes that are impossible to disentangle with empirical data.